Catalog number
FG-520.25
FG-520.50
FG-520.100
Application area
The TritaFetusGene® Multiplex QF-PCR Diagnostic Kit is a product utilized for the purpose of identifying aneuploidy in chromosomes 13 (also known as Pato syndrome), 18 (commonly referred to as Edward syndrome), 21 (more commonly known as Down syndrome), as well as in the X chromosome (associated with Turner, Klinefelter, and XXX syndromes) and the Y chromosome (linked to Jacobs syndrome). This particular diagnostic kit operates on the basis of a molecular technique known as Quantitative Fluorescent-Polymerase Chain Reaction (QF-PCR). In order to carry out this procedure, a total of 27 gene markers are examined, with the DNA used for analysis having been obtained from either amniotic fluid or chorionic villus sampling.
product performance principle
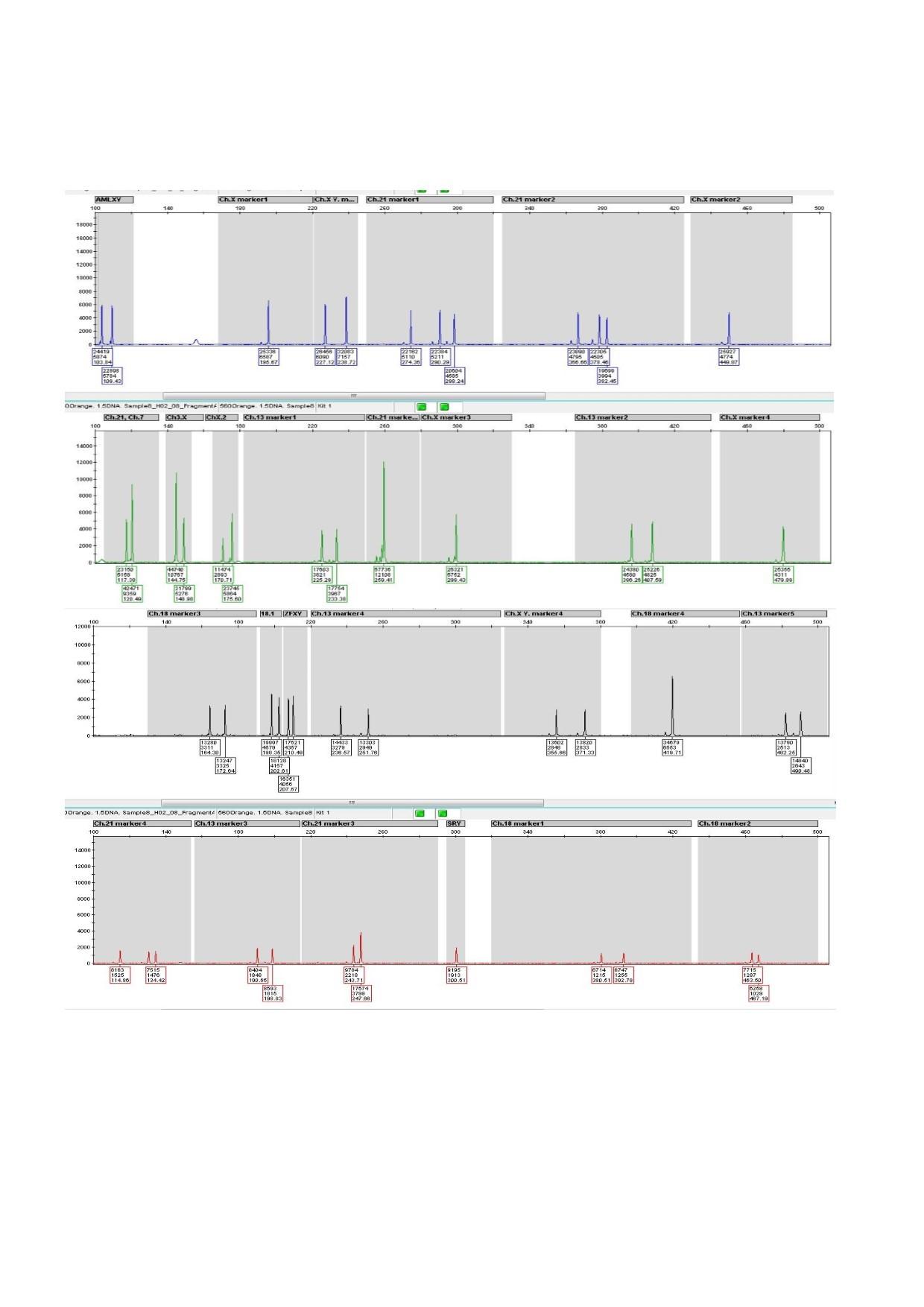
The TritaFetusGene® Multiplex QF-PCR Diagnostic Kit utilizes the Quantitative Fluorescent Polymerase Chain Reaction (QF-PCR) method, which is of great significance in the identification of non-mosaic trisomies. This particular kit amplifies 27 distinct regions of the genome, including short tandem repeats and various non-polymorphic markers, using primers that have been labeled with fluorescent dyes. The resulting PCR products, obtained through the utilization of the Genetic Analyzer device, are subsequently separated using the Capillary electrophoresis technique and then analyzed using specialized software. The determination of the relative size of each allele is achieved through the calculation of the height or area beneath the peaks.
In individuals who are considered to be normal, possessing two copies of each autosomal chromosome, the heterozygous alleles associated with a specific marker will generate two signals of equal area beneath the curve for each autosomal marker. Conversely, in individuals who suffer from trisomy, wherein there are three copies of the corresponding chromosome, ratios of 2:1, 1:2, or three signals 1:1:1 will be generated. In the instance of homozygous markers, only a single signal will be observed, leading to the designation of such markers as uninformative. Such markers are incapable of offering any utility in the identification of disorders pertaining to a number of chromosomes. This particular kit has been approved for use during three primary stages, which include:
-
DNA extraction
The TritaFetusGene® Multiplex QF-PCR Diagnostic Kit has been meticulously designed to ensure optimal performance when it is used with amniotic fluid and CVS chorionic villus samples. The template DNA required for this process is obtained using DNA extraction kits, such as the highly regarded Qiagen’s QIAamp DNA Blood Mini Kit.
-
DNA Amplification
This step is optimized in TritaFetusGene® Multiplex QF-PCR Diagnostic Kit with Applied Biosystem (Veriti) and BioRad (T 100) thermocycler.
-
Electrophoresis and results analysis
The PCR product derived from this kit possesses the capability to undergo analysis via Genetic Analyzer models ABI 3100, ABI 3739, ABI 3130 (XL), and ABI 3500 (XL) that are endowed with the capacity to detect 5 distinct colors. In order to analyze the outcomes of this instrumentation, GeneMapper® or GeneMarker® software is employed.
Main features of TritaFetusGene® Multiplex QF-PCR Diagnostic Kit
- Intended for prenatal determination of aneuploidies in chromosomes 13, 18, 21, X & Y
- based on Quantitative Fluorescent Polymerase Chain Reaction (QF-PCR) assay and capillary electrophoresis.
- Simultaneous amplification of 27 loci including STR, SD and SRY.
- Result analysis by professional softwares such as GeneMapper® or GeneMarker®
- Uses extracted DNA from amniotic fluid (AF) or Chorionic villus sampling (CVS)
- With %100 sensitivity and specificity
- Capable of detecting aneuploidies less than 5 hours after sampling.
- Manufactured by TritaGene Biotech Co. within quality management systems accredited to ISO 13485:2016 and ISO 9001:2015
Composition of TritaFetusGene® Multiplex QF-PCR Diagnostic Kit
TritaFetusGene® Multiplex QF-PCR Diagnostic Kit is available in 25, 50, and 100 test models. The table below displays the kit’s components and their values in various models of this product.
| The composition of tritaFetusGene® Multiplex QF-PCR Diagnostic Kit | ||||
| Tube/Cap Color | Vol. for 100 rxn (μl) | Vol. for 50 rxn (μl) | Vol. for 25 rxn (μl) | Kit Content |
| Amber/Yellow | 4 × 55 | 2 × 55 | 55 | TS-1 |
| Clear/Red | 4 × 375 | 2 × 375 | 375 | TS-2 |
| Clear/Blue | 4 × 70 | 2 × 70 | 70 | TS-3 |
|
* The kit contains a user’s Manual inside the package. |
||||
Technical specification of TritaFetusGene® Multiplex QF-PCR Diagnostic Kit
| Technical specification of TritaFetusGene® Multiplex QF-PCR Diagnostic Kit | |
| Kit Specifications and characteristics | Parameters |
| Multiplex PCR | Methodology |
| Quantitative Fluorescent PCR (QF-PCR) | Technique |
| Semi-quantitative | Type of Analysis |
| Short Tandem Repeats (STR); Segmental Duplications (SD); SRY gene | Target Sequence |
| DNA extracted from amniotic fluid (AF) and chorionic villus sampling (CVS) | Validated Specimen |
| More than %99 | Specificity |
| More than %99 | Sensitivity |
| -20 ± 5 °C | Storage |
| 6-FAMTM, VIC®, NEDTM, PET® | Dye |
| 30-40 ng/rxn | Recommended DNA Concentration |
| RIBO-prep nucleic acid extraction kit (AmpliSens®, Russia)
QIAamp® DSP DNA Blood Mini Kit (Qiagen, Germany) |
Validated Extraction Methods |
| BioRad (T 100), Applied Biosystem (Veriti), GeneAmp PCR System 9700 (Ramping rates: heating 0.8 ̊C/s, cooling 1.6 ̊C/s) |
Validated thermocyclers |
| Capillary electrophoresis | Detection Method |
| Applied Biosystems™ 3130/3130 xL Genetic Analyzer Applied Biosystems™ 3500/3500 xL Genetic AnalyzerABI 3100 Genetic Analyzer or ABI 3730 Genetic Analyzer |
Validated Genetic analyzers |
| 560 SIZER ORANGE (Devyser-Sweden) GeneScan™ 500 LIZ® Size Standard (Life Technologies-USA) GeneScan™ 600 LIZ® Size Standard (Life Technologies-USA) |
Size standard |
| GeneMarker®; GeneMapper® | Validated Softwares |