Cytomegalovirus (CMV)
Cytomegalovirus (CMV) is a member of the herpes virus family and one of the largest known pathogenic viruses. CMV, which belongs to the subfamily of beta herpesviruses, is the cause of a wide range of clinical syndromes from asymptomatic infections in healthy immunocompetent hosts to severe and fatal diseases in immunocompromised or immunosuppressed people.
CMV is an icosahedral virus with a size of 150-200 nm. The virus structure has four structural elements, which are the outer lipid envelope, tegument, nucleocapsid, and a nucleoprotein center that contains the virus genome. The viral envelope contains lipoproteins and structural proteins that play a role in the penetration of the virus into cells. Tegument also contains structural proteins including PP65; This protein is the most important diagnostic target in virus detection tests. The CMV genome is a 64 nm linear double-stranded DNA containing 235 kb, which contains non-overlapping open reading frames for over 230 proteins. One of the viral proteins is DNA polymerase, which plays a role in viral replication and is the most important target in common antiviral drugs.
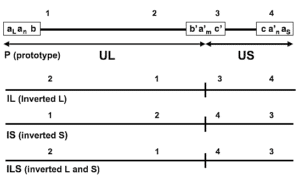
The human CMV has the largest genome in all herpesviruses and contains a high GC content. The virus genome contains unique long (UL), unique short (US), and repeat regions. Since each of the UL and US regions can be oriented in two directions, there will be four structural isomers for the virus genome. The virus genome contains 7 gene regions (A-G) that encode proteins necessary for viral function, DNA replication, nucleotide metabolism, and virus structure.
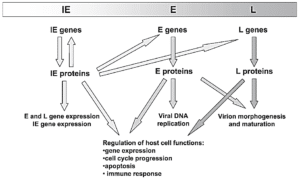
In the CMV life cycle, the viral genome is expressed and regulated in a regular cascade that leads to the production of three types of proteins including immediate-early (IE or α), early (E or β) and late (L or γ). IE genes such as UL122/123 are involved in the activation of viral genome expression. In addition, these genes impact host cell physiology through the regulation of cellular gene expression. The expression of E genes depends on the presence of IE proteins. E genes mostly express non-structural proteins, such as viral DNA replication factors, repair enzymes, and proteins involved in the immune evasion system. L genes encode proteins that play a structural role and participate in the formation of virus structure and
|
|
The CMV infection prevalence increases with age; In developing countries, the prevalence rate in children aged 1 to 5 years is 20.7% and in older adults, prevalence reaches 100%. CMV seroprevalence depends on various factors; These factors include geographic location, age, and socioeconomic. Seroprevalence is higher in developing countries and populous societies with economic problems. Infection with CMV happens mostly in childhood, by exposure to saliva, tears, urine, feces, breast milk, semen, and other body secretions of infected individuals. This virus can survive on certain surfaces for up to 6 hours, and it can also be transmitted through fomites. It is also possible to transmit the virus through tissue and organ transplantation or blood transfusion.
Clinical manifestations of CMV infection depend on the immune system. Primary infection with CMV is usually asymptomatic in healthy immunocompetent individuals. After a self-limiting period, CMV establishes latency in a wide range of body cells. These cells include endothelial cells, epithelial cells, smooth muscle and fibroblasts, and any part that the virus can carry by peripheral monocytes and circulating endothelial cells.
The primary infection by CMV leads to the production of CMV-specific antibodies (IgM and IgG) in the body. Reactivation of virus in a healthy immunocompetent triggers immunologic memory and leads to control of virus replication in the body. But on the other hand, the loss of CMV-specific T-lymphocyte cells (CD4 and CD8) in immunocompromised or immunosuppressed Patients can lead to uncontrolled replication of the virus and subsequent clinical symptoms. Patients with human immunodeficiency disease or AIDS, Patients receiving organ transplants, and Patients receiving hematopoietic stem cells whose immune system is defective or suppressed by the drugs, are more exposed to CMV-related clinical symptoms.
Primary infection with CMV during pregnancy can lead to intrauterine infection of the fetus and congenital CMV disease. Congenital CMV disease usually occurs in babies whose mothers are first infected with CMV during pregnancy, and in about 40% of cases, this infection is transmitted to the fetus. The risk of transmission is higher if the infection occurs in the first half of pregnancy. Clinical symptoms of congenital infection with CMV include petechial rash, jaundice with hepatosplenomegaly, neurologic abnormalities such as microcephaly and lethargy, chorioretinitis, and optic nerve atrophy, and prematurity and low birth weight. In 10-15% of cases, congenital infection in babies can lead to hearing loss.
There are various methods for laboratory diagnosis of CMV; These methods are generally classified into two categories: molecular techniques and non-molecular techniques. Non-molecular methods include viral culture, serology methods, antigenemia assay, and histopathology. Amplification of viral nucleic acid or NAT methods, which are mainly based on PCR, constitute a large part of the molecular methods of CMV diagnosis.
|

|
|
In the viral culture method, blood samples, urine, respiratory secretions, and other samples are inoculated into conventional-tube cell cultures such as MRC-5 human embryonic lung fibroblast and after incubation, the cells are checked for the presence of round and large cells which is a sign of cytopathic effect or the presence of CMV. The limitation of viral culture is its low sensitivity so, in a study examining liver biopsy samples, the sensitivity of this method was reported as 52%. The slow growth of CMV in the culture of human fibroblasts is another limitation of this method. On the other hand, cell and virus culture techniques require 1 to 4 weeks to observe the cytopathic effect.
The serology method and the detection of CMV-specific antibodies (IgM and IgG) are also used in the diagnosis of acute infection or previous infection with CMV; In this method, particles coated with CMV antigens are used, which identify CMV-specific IgM and IgG antibodies in the patient’s plasma or serum. Antigen-antibodies couple is detected using the horseradish peroxidase enzyme or other Antigen-antibodies detection methods. The serology method has several limitations; First, there is a time gap between the onset of clinical symptoms and the increase in IgM levels, which can lead to a delay in diagnosis and treatment. Second, in some people, IgM remains stable for a longer time after acute infection, and the detection of IgM does not mean active infection. Third, in people whose immune system is suppressed, the immune response is delayed and weak, which leads to false negative results in serology tests.
The Antigenemia method or direct detection of CMV antigens such as PP65 protein in neutrophil cells using monoclonal antibodies is a diagnostic method in immunocompromised or immunosuppressed individuals. The presence of PP65 is determined using immunofluorescence, immunoperoxidase, and other antigen detection methods. One of the limitations of this method is the need for a sufficient amount of neutrophils, which is not available in some people, such as leukopenic patients. also, with this method, only virus-infected cells are detected, and free viruses are not detected in biological body fluids such as plasma. Another limitation of this method is that the samples must be tested within a short period (6-8 hours) after collection. Therefore, this method cannot be useful for laboratories that work with a large volume of samples.
Nucleic acid amplification tests or NAT are used for rapid diagnosis of CMV in immunocompromised or immunosuppressed patients. These rely on the detection or amplification of CMV DNA in clinical samples. However, since CMV can exist latently in many nucleated cells, there is a risk that molecular methods will detect the inactive virus. Of course, the molecular method is much more sensitive than non-molecular methods. Among molecular methods, PCR-based methods are widely used in CMV diagnosis. The molecular method has made it possible to detect the virus in the early stages of infection when the viral load is low and the antibody level has not yet risen.
DNA is the target molecule in most of the molecular methods of CMV detection; but in some cases, RNA detection has been reported using the reverse transcriptase-PCR (RT-PCR) method. DNA detection is highly sensitive, and since the DNA molecule is stable in the sample, the delay in performing the test does not affect the DNA concentration. since viral RNA molecules will exist only during virus replication; RNA detection by using the RT-PCR method reduces the risk of detecting inactive or latent CMVs. Several gene targets on CMV DNA have been used for amplification using the PCR method, among which the DNA-polymerase gene and glycoprotein B gene can be mentioned.
Molecular methods can be done as qualitative (report as negative or positive) or quantitative (report as the amount of viral DNA) assays. Qualitative methods have high sensitivity, but their specificity is relatively lower than quantitative methods. In addition, qualitative methods cannot distinguish between active and inactive viruses. in addition, using qualitative methods, it is not possible to real-time check the responses of antiviral treatments. To improve the specificity and efficiency of molecular methods in clinical diagnosis, quantitative molecular methods or QNAT were developed that can report the amount of virus in the sample. Among these methods, we can mention the Real-Time PCR method based on TaqMan probes, which has high accuracy and sensitivity in CMV detection.
Quantitative Real-Time PCR or qPCR is a technique that uses PCR to amplify desired gene targets. This method uses fluorescent molecules to detect amplified products. During the reaction and amplification of nucleic acid, the amount of fluorescence radiation increases; As a result, it is possible to real-time analysis of PCR products quantitatively. Using non-specific fluorescent dyes (such as Cyber green) or using specific probes labeled with fluorescent dyes (such as TaqMan probes) are two common methods of viral genome detection. As a diagnostic tool, probe-based Real-Time PCR is used in the rapid and accurate detection of infectious viruses’ nucleic acid. To diagnose CMV infection, several commercial diagnostic kits have been designed and produced based on the Real-Time quantitative PCR method, which target and amplify virus genes such as DNA-polymerase gene and glycoprotein B gene.

