Fetal Sampling Methods
Having a healthy unborn baby is the most important concern of couples during pregnancy. The ultrasound method can detect fetal anatomy abnormalities. Screening of the maternal serum alone or along with ultrasound can also provide information about the risk of Down syndrome. However, to accurately diagnose the genetic condition of the fetus, fetal cells and DNA are needed. Prenatal diagnosis of chromosomal abnormalities is possible using cytogenetic and molecular techniques such as karyotyping, FISH, QF-PCR, and next-generation sequencing (NGS).
The requirement for performing these techniques is the safe foetal sampling and DNA extraction from fetal cells. The development of DNA-based diagnostic techniques has improved foetal sampling techniques including amniocentesis, fetal chorionic villus sampling (CVS), and fetal blood sampling (FBS). Foetal blood sampling is associated with late diagnosis and requires a high level of expertise. FBS is now rarely used to obtain a sample of foetal blood at a relatively late stage of pregnancy for rapid DNA analysis or globin chain synthesis. A major disadvantage of second-trimester amniocentesis is that the results are available relatively late in pregnancy (after 16 weeks’ gestation). An earlier alternative is CVS which can be performed in the first trimester of pregnancy.
Amniocentesis
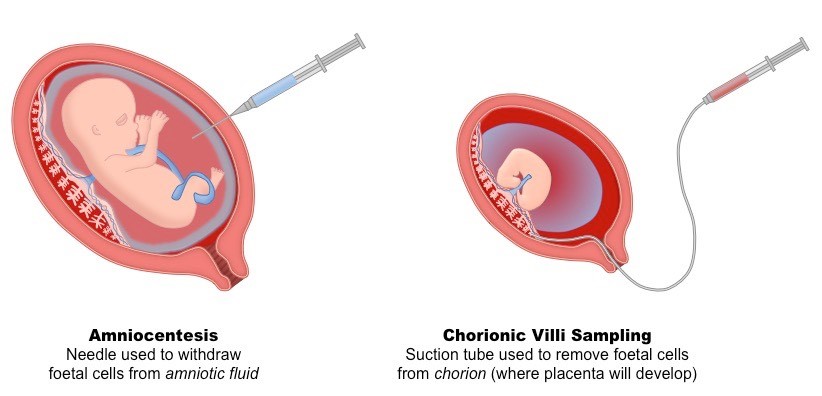
One of the most common procedures for detecting prenatal abnormalities is amniocentesis which is often offered to women over 35 because they have a higher risk of having a fetus with chromosomal abnormalities. Amniocentesis can usually be done when a woman is pregnant with twins or even more fetuses. Amniocentesis, or sampling from amniotic fluid in the second trimester of pregnancy, was first introduced in the 1960s and is still used as one of the most common invasive techniques for prenatal diagnosis of fetal genetic abnormalities. The global acceptance of this technique increased with the improvement of safety with the use of ultrasound guidance. Amniocentesis is usually performed after 16 weeks’ gestation. The procedure includes the insertion of a sterile needle through the abdominal wall into the amniotic sac under ultrasound guidance, and a 15-20 ml sample of fluid is withdrawn and sent to a lab for testing. The fluid contains cells that have been shed by the fetus. cell culture is needed to analyze the fetal chromosomes. Before the procedure, ultrasonography is done to evaluate the heart of the fetus, confirm the length of the pregnancy, locate the placenta and amniotic fluid, and determine how many fetuses are present.
Amniocentesis, similar to CVS, has decreased in frequency with increased utilization of cell-free fetal DNA screening. It remains the only diagnostic test available in the second or third trimesters of pregnancy and may be performed at any gestational age after 15 weeks. It is often a safe procedure for both mother and fetus. Not only, amniocentesis is used to evaluate genetic disorders, but it is also performed to investigate the presence of intra-amniotic or fetal infection via culture or polymerase chain reaction or the neural tube defects by measuring amniotic fluid alpha-fetoprotein and acetylcholinesterase. Nevertheless, Occasionally, the amniotic fluid contains blood from the fetus. Such blood may increase the alpha-fetoprotein level, making the results hard to interpret.
The risks associated with performing amniocentesis at 16 weeks’ gestation are reported to be between 0.5-1%, and this rate is even lower in experienced centers. In addition, the introduction of real-time ultrasound technology, allows the operator to continuously monitor the needle tip and avoid direct damage to the fetus. Amniocentesis complications are more common at earlier gestational ages. Pregnancy loss attributed to amniocentesis is approximately 1 in 900 on most recent estimates. Amniocentesis in the first trimester of pregnancy is associated with a higher risk of miscarriage than in the second trimester. Amniotic fluid may not have enough DNA to perform molecular tests, in which case cell culture is necessary, and this issue delays the diagnosis. Postponing the diagnosis in cases that require termination of pregnancy will not be acceptable.
If women have Rh-negative blood, they are given Rho(D) immune globulin after the procedure to prevent them from producing antibodies to Rh factor. A woman with Rh-negative blood may produce these antibodies if the fetus has Rh-positive blood and the blood comes into contact with her blood (Rh incompatibility), as it may during amniocentesis. These antibodies can cause problems in a fetus with Rh-positive blood. The injection is not needed if the father also has Rh-negative blood because in such cases, the fetus always has Rh-negative blood.
|
Chorionic villus sampling (CVS)
Chorionic villus sampling (CVS) is a procedure in which a gynecologist removes a small sample of the chorionic villi, tiny projections that make up part of the placenta. CVS is performed between 10 and 14 weeks of pregnancy. It is not recommended before 9 weeks of gestation as it increases the risk of limb deformities. CVS allows for earlier prenatal diagnosis, subsequently decreasing the time of uncertainty and allowing for earlier and safer pregnancy termination if needed. Also, early detection of an abnormality may give the couple more time to prepare for the birth of a child with special medical needs.
CVS was originally carried out using either forceps or a catheter passed through the uterine cervix (transcervical). More recently, transabdominal CVS has been introduced in clinical practice; This method involves obtaining a biopsy of the placenta using a needle inserted through the abdominal wall under ultrasound guidance to insert the catheter or needle and suction out the tissue sample with a syringe without entering the amniotic sac. Before CVS, ultrasonography is also done to determine whether the fetus is alive, confirm the length of the pregnancy, check for obvious abnormalities, and to locate the placenta. Finally, the sample is sent to a clinical lab for analysis.
CVS has decreased in frequency with the recently increased uptake of cell-free DNA screening. It remains the only diagnostic test available in the first trimester and allows for diagnostic analyses including fluorescence in situ hybridization (FISH), karyotype, microarray, and molecular testing such as QF-PCR and gene sequencing.
The great advantage of CVS is the possibility of sampling in the first trimester of pregnancy, which is within the limits laid down by abortion law in many countries. Also, this method reduces the emotional stress caused by late diagnosis and the risk of complications due to the termination of pregnancy. CVS also has technical advantages; Using this technique, more embryo cells are obtained than other sampling methods.
The risk of fetal loss due to CVS has been reported between 0.5-4.5% in various studies. Performing CVS requires a dedicated team, and if performed by an experienced operator, the risk of fetal loss reaches 1.5-1%. There is no evidence of increased obstetric complications associated with CVS. Vaginal spotting or bleeding is one of the most common complications caused by CVS (1-4% of cases) and it is mostly seen when transcervical CVS is performed (up to 20% of cases). Because CVS involves puncturing the amniotic sac, it may cause oligohydramnios. Intrauterine infection and amniotic fluid leakage are other complications associated with this technique.
Nevertheless, the main disadvantage of CVS is that approximately 1-2% of CVS results may reflect confined placental mosaicism rather than true fetal chromosomal abnormalities. Confined placental mosaicism may increase the risk of having a small-for-gestational-age infant.
After chorionic villus sampling, women with Rh-negative blood that do not have antibodies to Rh factor are given an injection of Rho(D) immunoglobulin to prevent them from producing antibodies to Rh factor. A woman with Rh-negative blood may produce these antibodies if the fetus has Rh-positive blood and it comes into contact with her blood (Rh incompatibility) during chorionic villus sampling. These antibodies can harm the fetus. If the father also has Rh-negative blood in such cases, the fetus always has Rh-negative blood and no injection is needed.
The risks of chorionic villus sampling are comparable to those of amniocentesis. Rarely, the genetic diagnosis is unclear after chorionic villus sampling, and amniocentesis may be necessary. In general, the accuracy of the two procedures is comparable.

